Antigen Binding, Presentation and T Cell Activation
Background
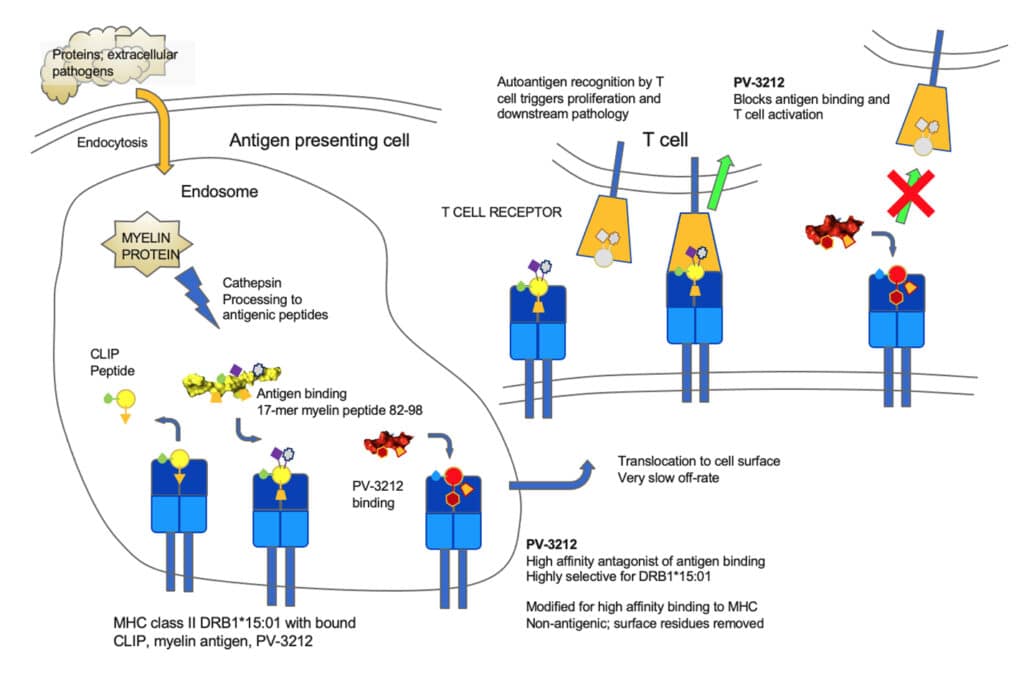
The immune system recognizes and defends against pathogens by a multi-step process initiated in antigen presenting cells (APCs). Foreign extracellular pathogenic proteins are taken up by APCs and transported to acidic endosomal compartments where a pathogen is degraded by cathepsin enzymes into fragments. MHC class II molecules enter the endosome in complex with an invariant chain protein that is cleaved to form a peptide (CLIP) that remains in the MHC binding groove where it can be exchanged for other peptides (including antigenic ones) that then are presented in the MHC-antigen context to TCRs. Those that are recognized as foreign trigger T cell proliferation and destruction of infected cells.
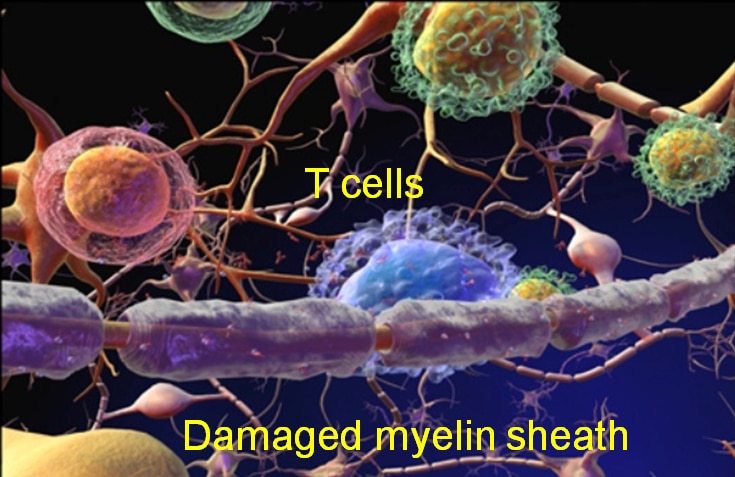
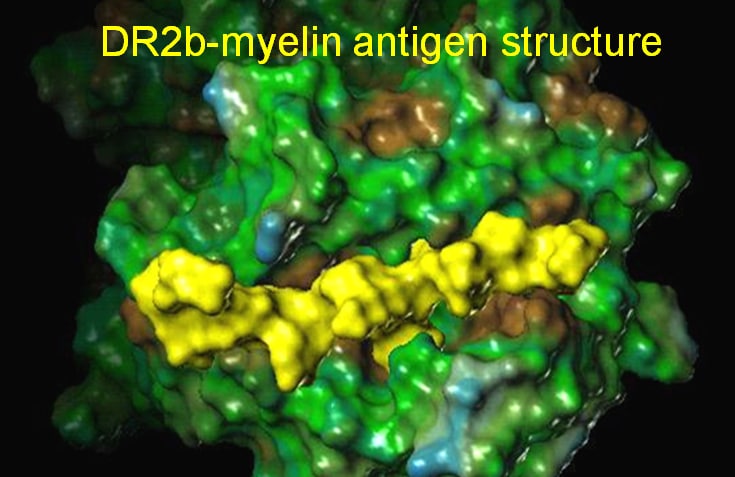
In the case of an autoimmune disease, such as multiple sclerosis, fragments derived from the nerve insulating protein myelin exchange with the CLIP peptide. This is a process catalyzed by another MHC molecule, HLA-DM, and occurs in a rapid equilibrium process that loads the myelin peptides into the DRB1*15:01 binding site. The complex then translocates to the cell surface where a pH change makes the binding of the antigenic peptides essentially irreversible and allows the complexes to engage with autoreactive T cell receptors (TCR). As a result of a molecular mimicry, the natural myelin peptide is mistaken for a pathogen, and autoreactive T cells proliferate and initiate a process to destroy the myelin nerve insulation as if it were a foreign pathogen, leading to the autoimmune disease pathology.
In the immune system, DR15 is a key component responsible for immune protection from certain foreign pathogens. DR15 interacts with fragments of the myelin insulation on nerves, but in MS disease, this interaction leads to activation of autoreactive T cells that go on to damage the myelin insulation, leading to the disease pathology. Provid’s inhibitors block this critical step, preventing autoreactive T cell activation, and have demonstrated potent efficacy in animal models of MS and in human cells from MS patients.
PV-3212 First Drug Candidate Blocking DR15
PV-3212 is a potent inhibitor of MHC DR15 binding that is stable toward cathepsin degradation and binds with high (picomolar) affinity to DR15 but has modified residues that do not permit T cell binding to the complex. PV-3212 acts as a pure antagonist of antigen binding and inhibitor of antigen presentation. In the absence of an MHC-antigen-TCR interaction, the autoimmune process is disrupted.
PV-3212 is a small molecule, designed to be highly selective for DR15 and to have minimal inhibition of other MHC class II molecules. This unique mechanism promises to disrupt the autoimmune disease process related to DR15 and to inhibit MS pathology without compromising other normal immune processes.
No other autoimmune disease drugs have been developed via this mechanism
MHC Genetic Association
Provid’s approach targets MHC Class II DR15, the most important gene target in the autoimmune disease process in MS. There are an estimated 1 million MS patients in the US and 2.5 million worldwide. MS is genetically linked with the MHC class II molecule HLA-DRB1*15:01 (DR15). DR15 is carried by a majority of MS patients and binds and presents antigenic myelin fragments to autoreactive T cells, triggering the autoimmune response that damages myelin insulation on nerves, leading to MS disease symptoms and disability. Provid designed PV-3212 to bind tightly and selectively to DR15, blocking antigen binding. PV-3212 has been shown to disrupt the autoimmune process in MS animal models and in human cells from DR2+ patients.
HLA-DRB1*15:01 (DR15) and Autoimmune Disease
PV-3212, the first genetically targeted drug for MS
PV-3212 blocks antigen binding specifically to DR15, inhibiting T-cell activation without blocking other normal immune responses. This specificity is an important safety factor that is advantageous vs. existing MS drugs that have serious safety concerns due to their non-specific mechanisms. The initial Provid lead, PV-267, was demonstrated to be effective in MS models in DRB1*15:01 transgenic mice in studies performed by our collaborator, Prof. T. Forsthuber, at UT San Antonio. Provid’s 2nd generation analog PV-3212 binds to DR15 with high affinity and is more potent than PV-267 in human cells from DRB1*15:01-positive MS patients. PV-3212 was chosen as the 2nd generation drug candidate for its high metabolic stability and superior pharmacokinetics.
MHC Class II HLA DR15 inhibition as a platform for autoimmune diseases
- Precision medicine concept: treat patients who carry disease-associated MHC with inhibitors of MHC-antigen binding
Autoimmune diseases are genetically associated with specific MHC class II molecules
DR15 (DRB1*15:01/DRB5*01:01)
- Multiple Sclerosis
- Goodpasture’s disease
- Prodromal Parkinson’s
- D. Sulzer; D. Agalliu (Columbia) Prodromal Parkinson’s is linked to α-synuclein antigen binding to DR15 (Garretti et al., 2023, Neuron 111, 3397–3413)
- Lupus nephritis, PR3-ANCA
DR4 DR1
- Rheumatoid arthritis
- Celiac disease
- Type 1 diabetes
DR4
- Pemphigus vulgaris
DR3
- Lupus (SLE)
Unique mechanism of action
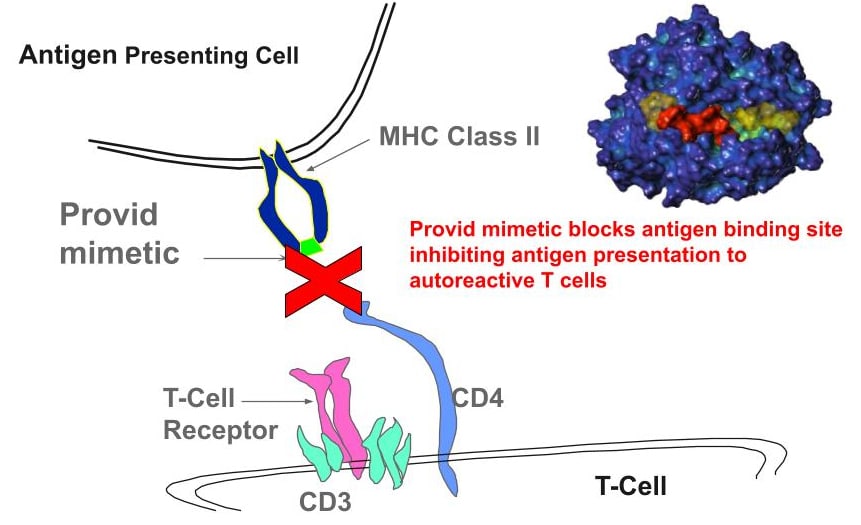
The unique mechanism of action of PV-3212 is to block the antigen binding site of DR15. PV-3212 binds with picomolar affinity to DRB1*15:01 and nanomolar affinity to the co-expressed DRB5*01:01. PV-3212 competitively antagonizes autoantigen binding, blocking T cell activation and downstream autoimmune disease pathology. The activity of PV-3212 has been demonstrated in human cellular immunology models using cells from DR15-positive MS patients stimulated with myelin antigen. Inhibition of DR15 has also been shown with other compounds in the series (e.g., the initial lead PV-267) to be effective in transgenic mouse models of MS (EAE model). Validating the MHC targeting concept further, efficacy has also been shown in models of Goodpasture’s disease, a rare DR15-associated kidney disease that is triggered by a collagen-related antigen.
Science: Targeting MS Disease Mechanism
In MS, the myelin nerve insulation is attacked by the patient’s own immune system
Antigen binding to HLA-DRB1*15:01 (DR2b; DR15) activates autoreactive T cells triggering downstream pathology
All currently marketed drugs target downstream effects, not this primary disease mechanism
Provid Concept:
- BLOCK DR15 = PREVENT ANTIGEN BINDING = DISRUPT MS
- Early MS, RRMS, block progression to SPMS
- Selective DR15 blockade promises high efficacy and superior safety in DR15+ patients without compromising normal immune system