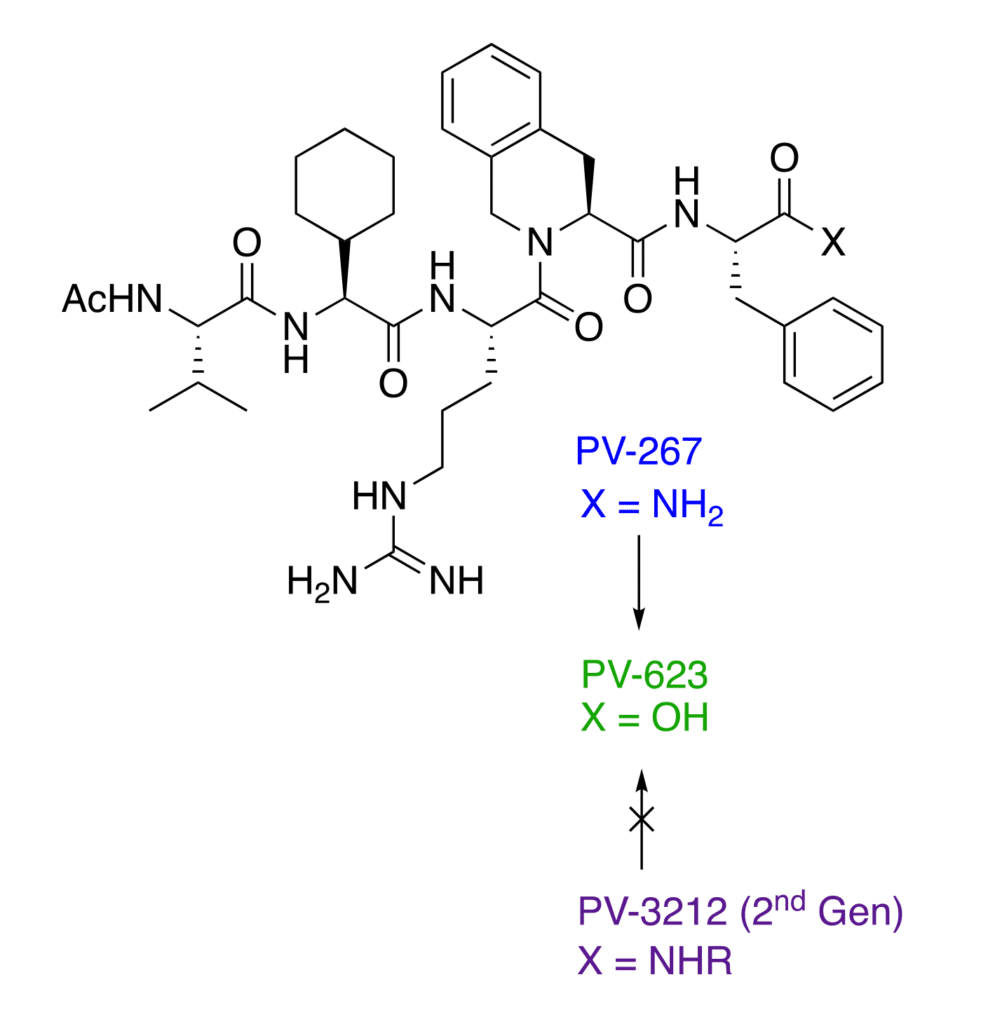
PV-3212 is a novel, small molecule drug candidate for the treatment of multiple sclerosis (MS) being developed by Provid Pharmaceuticals. It is a potent and specific inhibitor of the major histocompatibility class II molecule HLA DRB1*1501 (“DR15”), the immune target genetically associated with MS. Specific inhibition of DR15 promises high efficacy in treating MS in the population carrying this gene without compromising normal immune responses. PV-3212 is a novel, second-generation drug having potent activity, high metabolic stability, and 20+ years of patent life.
Therapeutic Strategy
Precision medicine targeting the DRB1*1501 MS patient population
PV-3212 is anticipated to be the first precision medicine for MS, designed to treat exclusively the 40-60% of MS patients who carry the DR15 gene. PV-3212 will be profiled as a specific, targeted therapy for MS in these patients based on (1) its unique mechanism of action addressing a fundamental step in the autoimmune disease process and (2) the expected safety benefits of this targeting.
The comprehensive understanding of the genetic nature of autoimmune diseases including multiple sclerosis has been developing over several decades. However, no inhibitor molecules with the potential to selectively block the disease-associated MHC have been available until the Provid work. It is anticipated that this breakthrough in personalized or precision medicine represented by PV-3212 will become a new standard of care for MS patients and a trailblazer for the treatment of other autoimmune diseases
Antagonist PV-3212 Design
Drug Design and Optimization
PV-3212 Drug Candidate
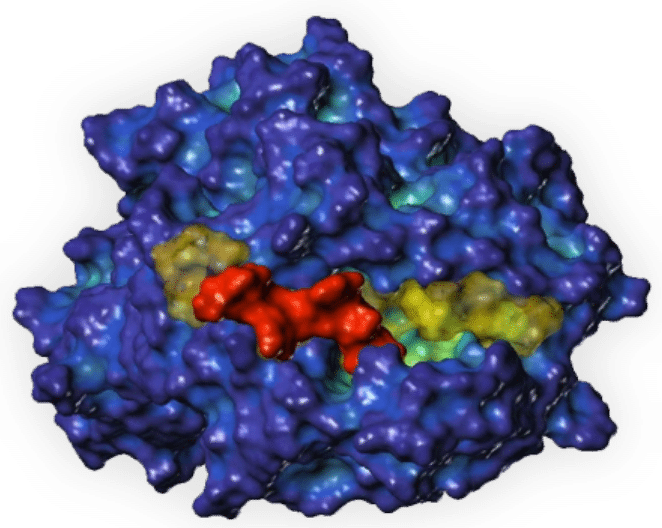
The crystal structure of DRB1*15:01 in complex with the myelin antigen fragment MBP 82-98 (peptide sequence DENPVVHFFKNIVTPRT) shows binding interactions across the MHC groove, with key anchor residues at p1 (V), p4 (F), and multiple interactions with the peptide backbone.
Drug Design Approach
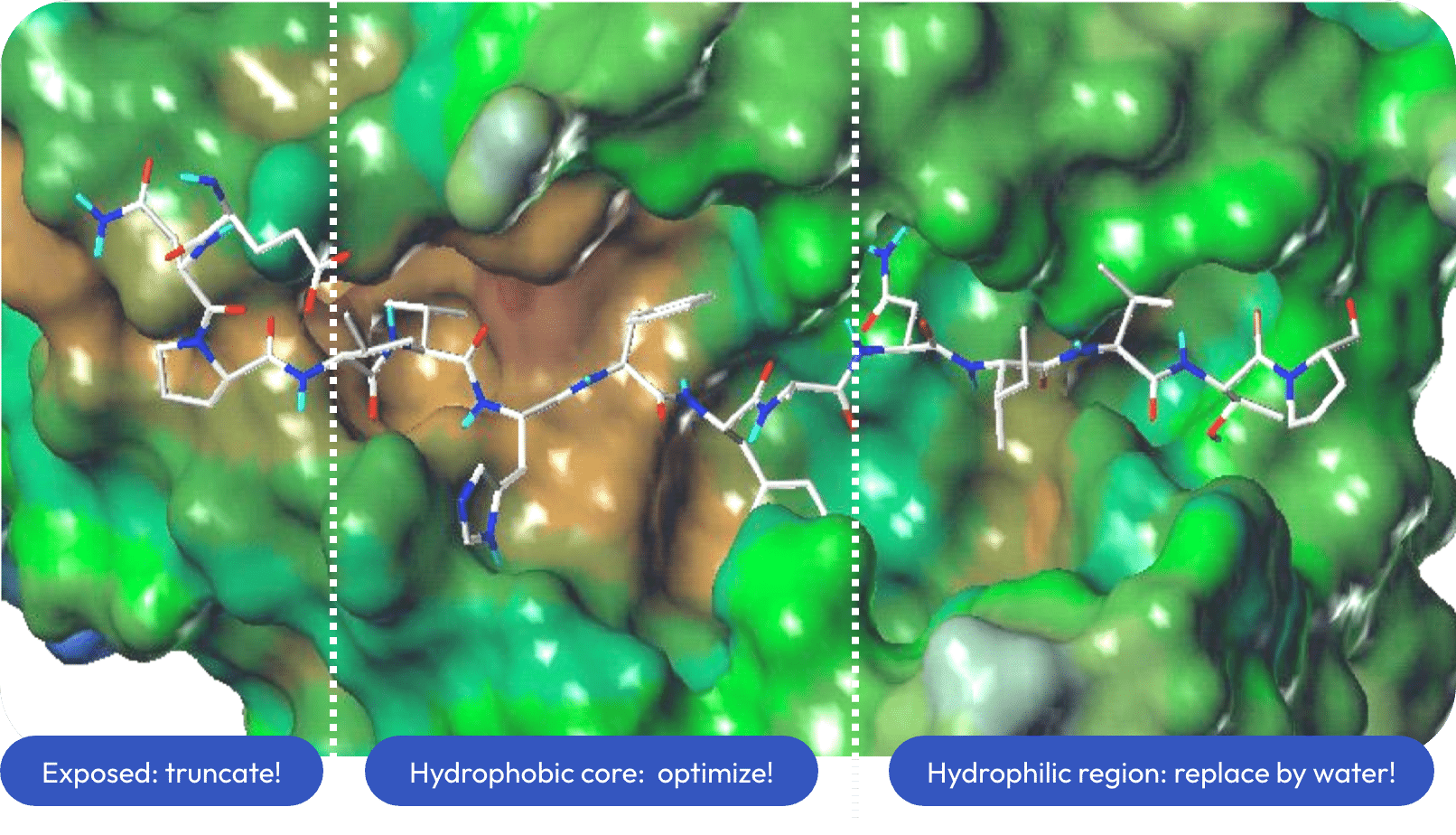
Truncation to P1-P4 core
Provid’s design focused on the hydrophobic MHC region that binds peptides from residues p1 through p4 and includes deep binding pockets (anchor positions). For our inhibitor design, residues outside this four amino acid core were truncated then the individual side chains were optimized.
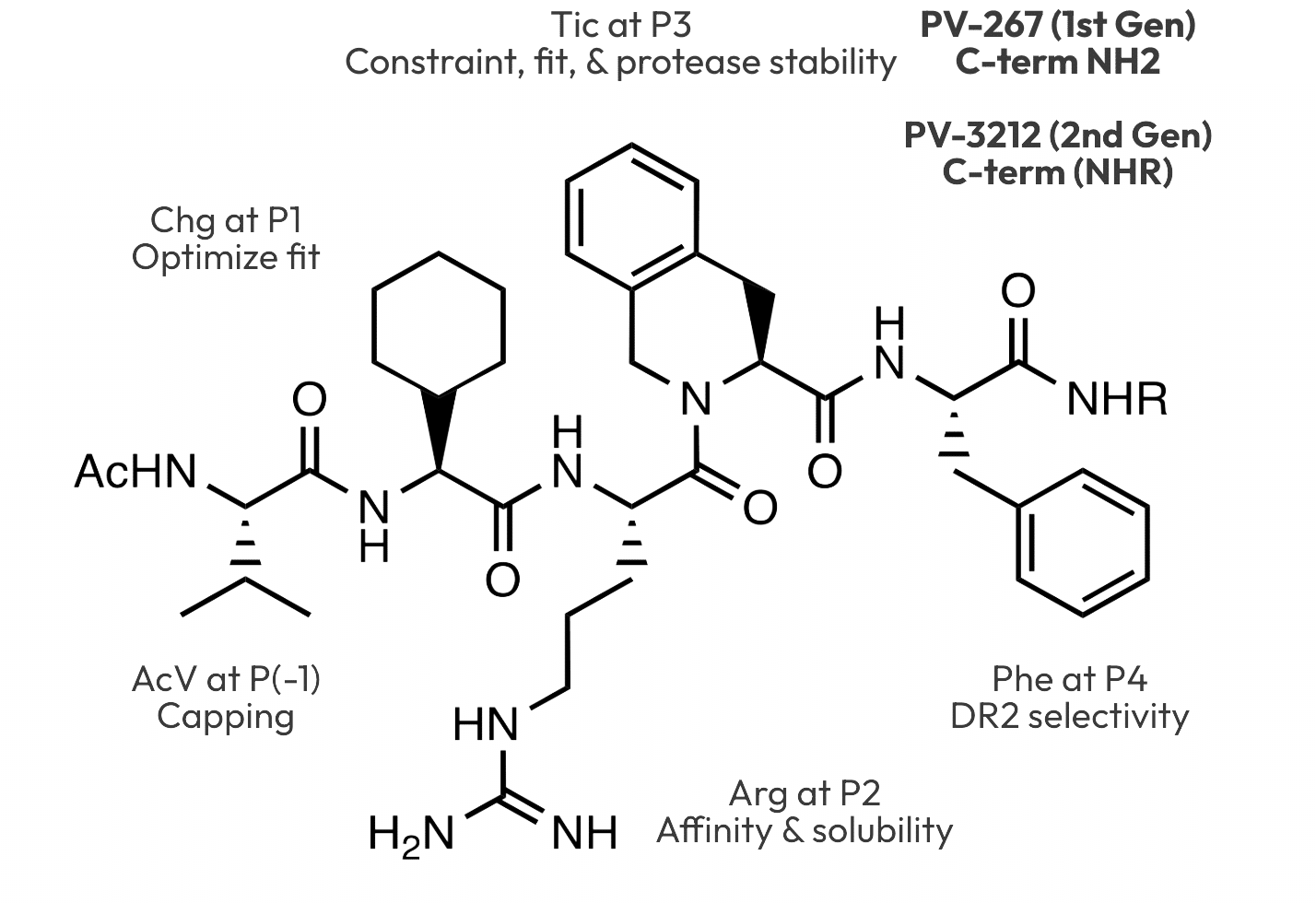
P1 Optimization
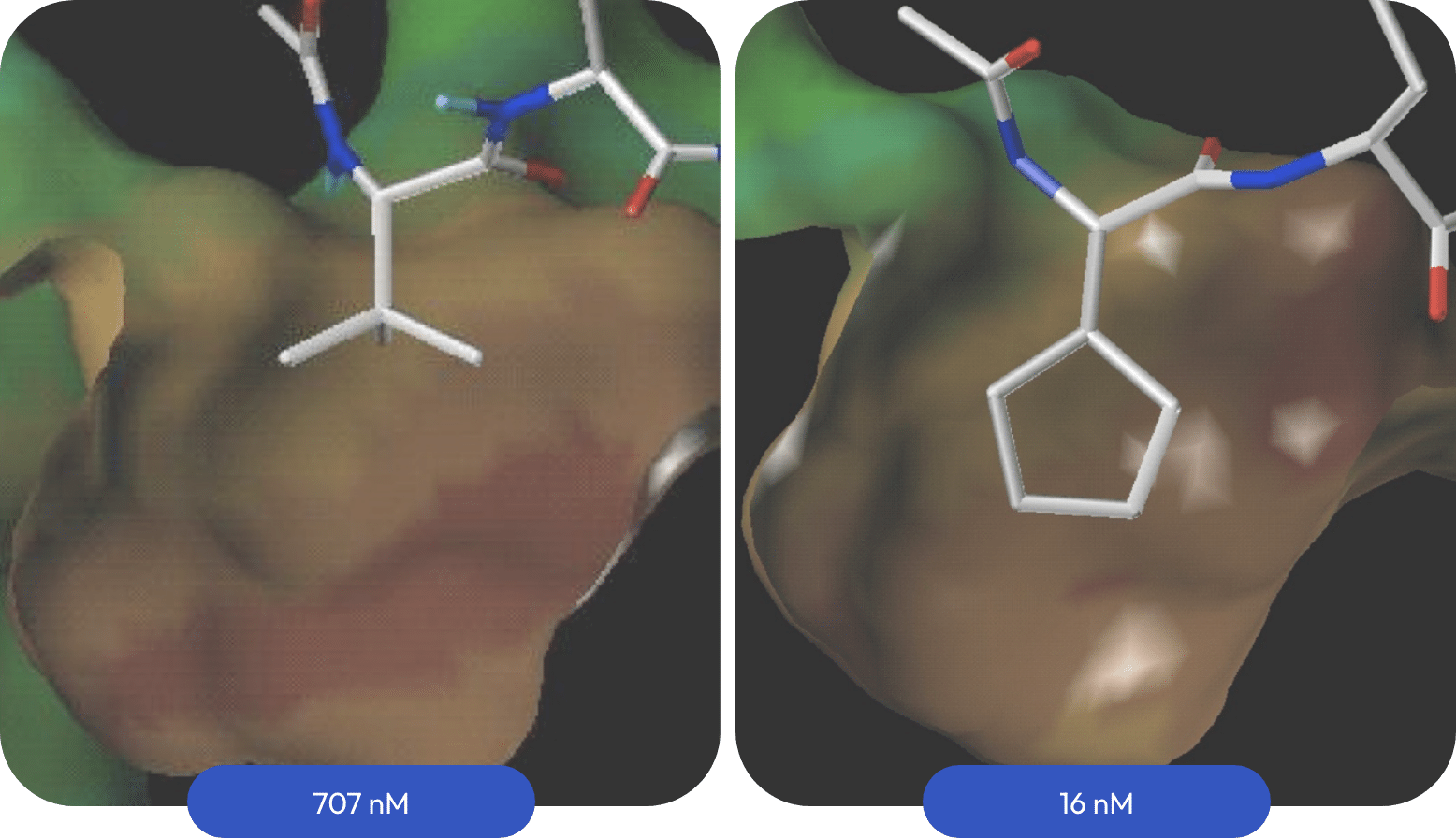
The myelin peptide has a valine at p1 that only partially fills the hydrophobic pocket. A reference sequence with p1 valine binds to DRB1*15:01 with 707 nM affinity. Filling the pocket optimally with cyclopentyl- or cyclohexylglycine instead of valine at p1 increased binding significantly. The p2 residue on the binding surface was optimized to arginine. Adding a p(-1) valine to shield the p1 backbone amide from solvent and combining this with optimized p2-p4 groups generated potent leads.
Backbone Stabilization
Antigen binding occurs within the endosomal compartment where cathepsin enzymes are active to degrade protein antigens to the shorter sequences that bind the MHC. Such enzymes are also able to cleave potential inhibitors if they are all natural amino acids. The use of cyclohexylglycine at p1 and replacing p3 phenylalanine with a constrained cyclic Tic variant prevented cleavage of the adjacent residues.
Remove TCR-recognition groups
DR15 Selectivity via unique P-4 pocket
The P-4 pocket in DRB1*15:01 is larger than other HLA-DR binding sites that have either a lysine or arginine group at position 71 in the beta chain of the MHC. In DRB1*15:01, this position 71 is a small alanine group, thus enlarging the binding pocket. We incorporated phenylalanine at p4 to exploit this larger pocket, producing inhibitors with high specificity for DRB1*15:01.
C-capping to Enhance Pharmacokinetics
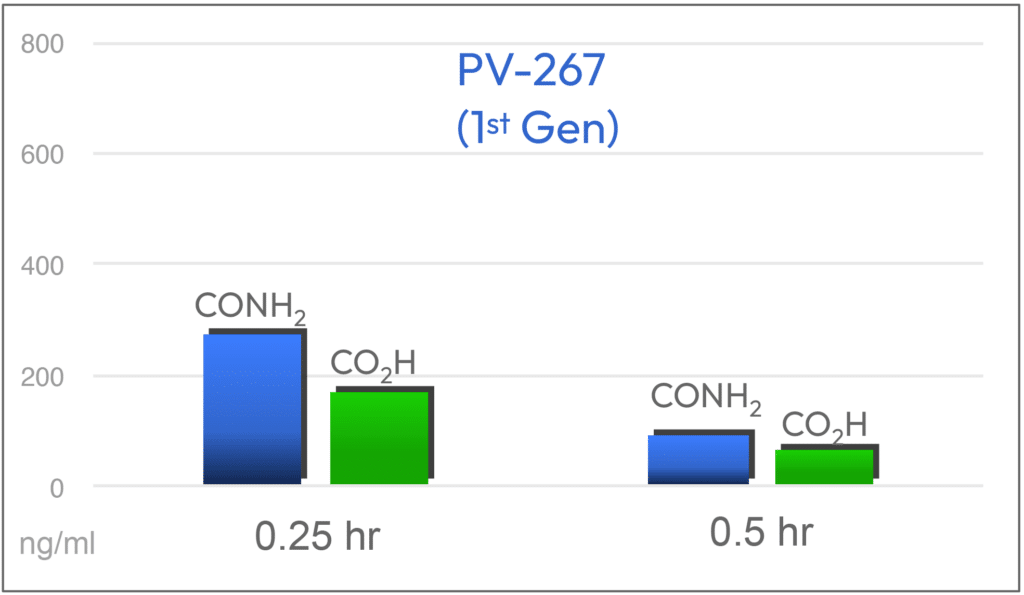
The C-terminal amide group (CONH2) at p4 on the first generation compound PV-267 was shown to be metabolized to the corresponding acid (COOH). In binding and cellular assays, the acid was much less potent than the amide. Based on this result, a series of substituted amides (CONH-R) were designed and found to be resistant to metabolism to the acid.
Combining design features results in drug candidate PV-3212
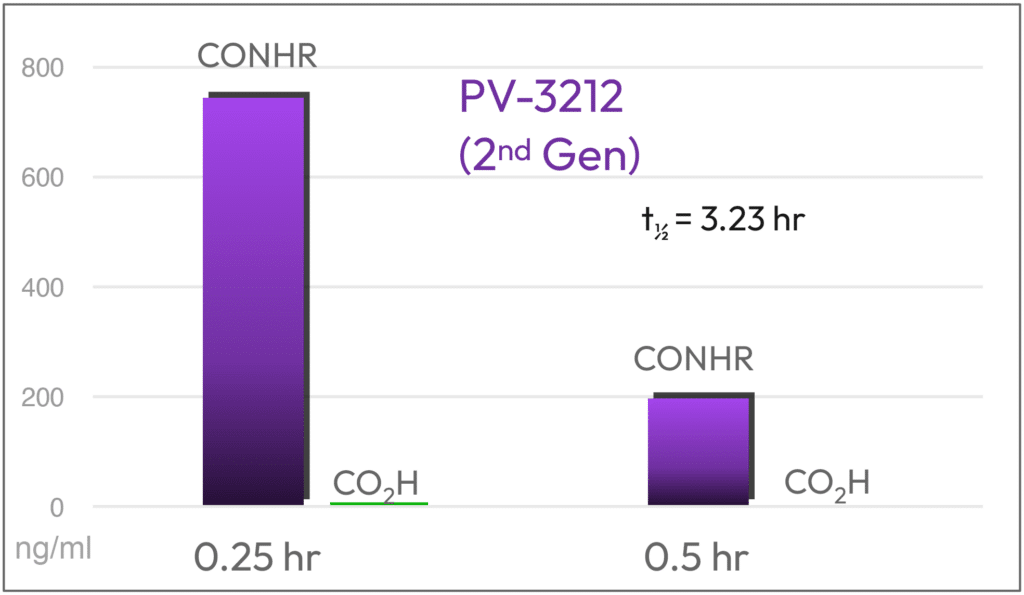
The combination of design elements produced PV-3212, a picomolar binding ligand that is not metabolized significantly, has higher plasma levels, and a longer half-life than the first generation compounds.
PV-3212 Preclinical R&D
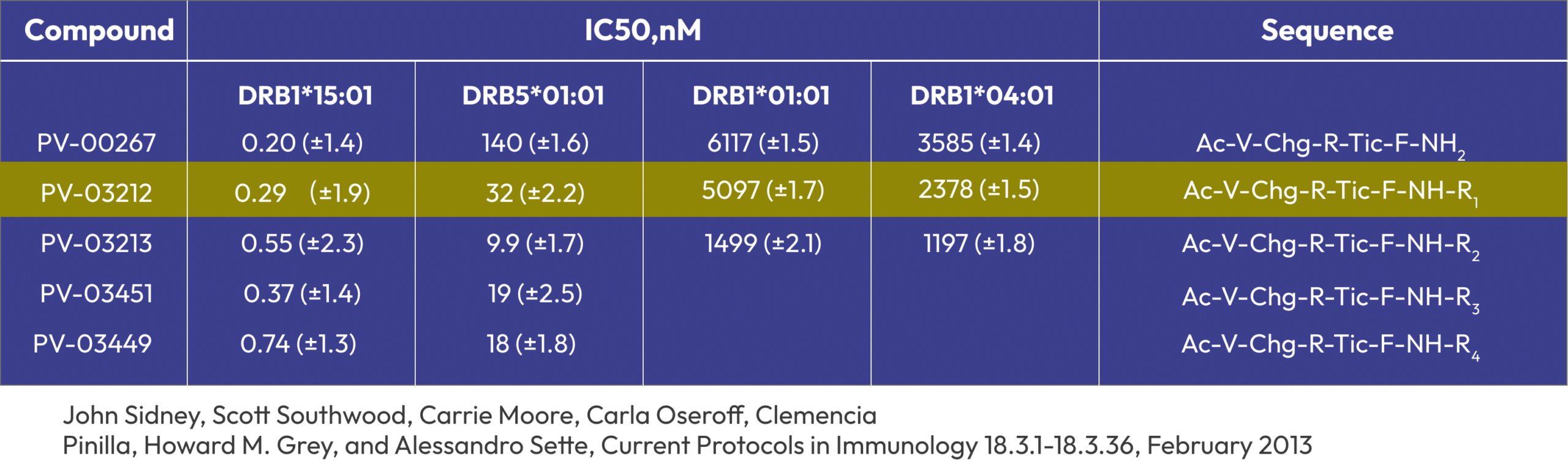
Binding Results (picomolar binding/JS La Jolla Inst Immunol)
PV-3212 and a series of homologous C-terminal variants were evaluated for binding affinity to HLA-DRB1*15:01, DRB5:01:01 and DRB1*01:01 by John Sidney in the Sette group at the La Jolla Institute of Immunology.
Human cells
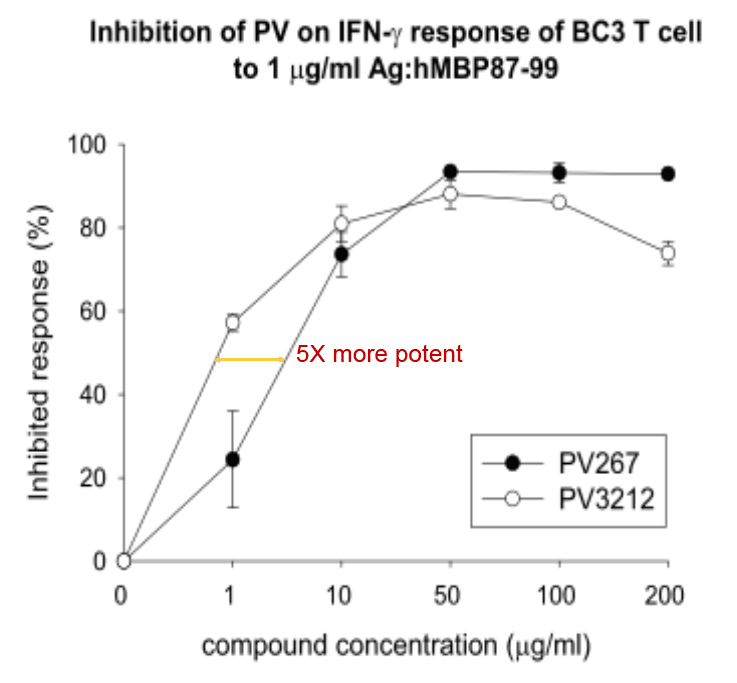
High potency inhibition of IFN-γ response to MBP87-99 antigen
2nd Generation: Ac-V(Chg)R(Tic)F-NHR
PV-3212; PV-3213; PV-3449; PV-3451
Metabolism and PK
Parent and Acid Metabolite (Mouse PK; 10 mg/Kg) PV-267 (1st Gen) vs PV-3212 (2nd Gen)
PV-3212
Metabolically Stable
2.7X Higher plasma levels
5X More potent (Hu Cells)
DR15 Humanized mouse and EAE model results
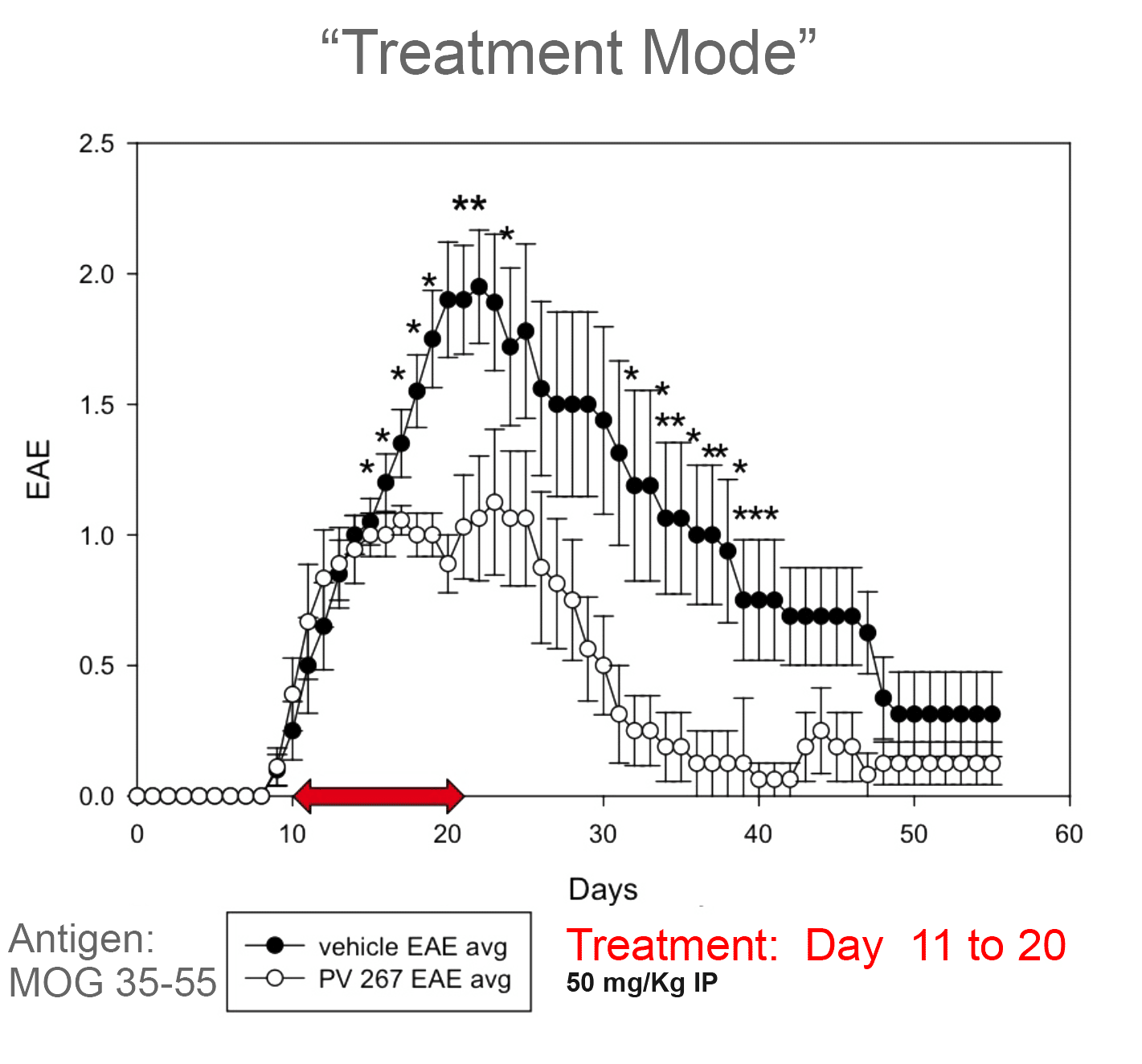
Provid’s DR15 inhibitor has been evaluated in an EAE model (a standard model of MS) but using transgenic mice in which the mouse class II has been replaced by the human DRB1*15:01 to mimic human in vivo responses. In this model, EAE is induced using MOG or myelin peptides and doses of PV-267 (first generation inhibitor) were administered either at the time of immunization (prevention mode) or at the time of disease onset (treatment mode). PV-267 showed potent inhibition of EAE clinical scores throughout dosing in the prevention mode. Furthermore, in the more challenging treatment mode, PV-267 was administered for 10 days after disease onset and resulted in impressive suppression of EAE.
High Efficacy in EAE
DR15 Transgenic Mice
Small Molecule Inhibitor of Antigen Binding and Presentation by HLA-DR2b as a Therapeutic Strategy for the Treatment of Multiple Sclerosis
Niannian Ji, Animesh Somanaboeina, Aakanksha Dixit, Kazuyuki Kawamura, Neil J. Hayward, Christopher Self, Gary L. Olson and Thomas G. Forsthuber J Immunol October 11, 2013, 1300407.
API Synthesis
PV-3212 is a modified pentapeptide/mimetic having unnatural amino acids and end-caps to enhance binding to the DR15 binding site and to stabilize the structure against degradation by cathepsin and plasma proteases. The synthesis has been shown to be feasible by solid-phase peptide synthesis or by solution methods. PV-3212 was selected to replace the initial lead PV-267 as the development candidate based on its higher metabolic stability and increased potency.
Formulation and oral bioavailability
Efficacy for PV compounds has been demonstrated in animals by IP or SC injection and preliminary evidence of oral bioavailability for PV-267 has been obtained from novel oral formulation technology developed by Enteris Biopharma. PV-3212 could be developed initially as an injectable and further developed as an oral formulation as appropriate for the clinical need and pharmacological profile.
Clinical Development
Clinical Development of PV-3212
A comprehensive understanding of the genetic nature of autoimmune diseases and their association with MHC class II has been developing over several decades. However, no inhibitor molecules with the potential to selectively block a disease-associated MHC have been available until the Provid work on DR15 inhibitors. It is anticipated that this breakthrough in personalized/precision medicine represented by PV-3212 will become a new standard of care for MS patients and a trailblazer for the treatment of other autoimmune diseases. The clinical development strategy for PV-3212 is built around this fundamental new mechanistic concept.
Early clinical trials (Phase Ib mechanistic proof-of-concept in MS patients)
The mechanism of action of PV-3212 described above suggests criteria for patient selection, efficacy evaluation, diagnostic and biomarker analyses as part of the clinical trial and therapeutic strategy, including the following:
- HLA typing of MS patients prior to entry into drug trials or clinical treatment. Such assays are commercially available in a number of formats and widely used. Only DR15 patients will be treated with PV-3212.
- Selection of DR15-patients with early MS (prodromal, CIS/RIS or RRMS) for initial efficacy studies. Since PV-3212 blocks the initial antigen binding and T cell activation, patients with early disease or disease in relapse are expected to give the strongest clinical readout in the short term. In later phase II/III studies, inhibiting the progression from early MS can be evaluated as well. Demonstration of efficacy in these DR15 patients could lead to first-line therapy designation for this population.
- Inhibition of DR15-restricted T cell activation will be assessed in patients treated with PV-3212 using blood samples and ex-vivo antigen-induced cytokine production as a biomarker of efficacy. This has been modeled on studies in DR15 transgenic mice and human cells from DR15+ MS patients. Clinical protocols will be developed further in collaboration with Prof. Lawrence Steinman at Stanford (Provid SAB, clinical chair). Such studies are initially intended for proof-of-concept studies to define dosing for PV-3212 for phase II clinical trials but may be used to monitor response in patients.
- Evaluation of brain lesions by MRI or other biomarkers including neurofilament light chain (NfL) levels as indicators of disease activity/treatment response. This model has been shown to be a good indicator in multiple forms of MS.
Phase II studies
- In addition to continuation of the biomarker studies from phase Ib, phase II would include standard Gd-enhanced MRI lesion studies as a surrogate of efficacy. The number and frequency of relapses in RRMS and evaluation of disease progression using the Expanded Disability Status Scale (EDSS) would also be pursued.
Patents
1st Generation Patents
- Issued US patents 7,439,231 (composition of matter), 8,222,215 (use), 8,598,312 (composition of matter).
2nd Generation Patents (composition of matter)
US Patent Application No. 63/163,172 filed March 19, 2021; Published as US 20240158439-A1 on May 16, 2024; WO2022/197499 published September 22, 2022.
Exclusivity at least through 2046 in US.